Saturated steam has a higher heat transfer coefficient than super
heated steam. Use saturated steam for indirect heat transfer applications.
Latent heat of evaporation forms most of the energy content of steam. For
indirect heating applications in a process, it is this latent heat of evaporation in
steam that is actually utilised. The condensate which leaves the plant
equipment contains a portion of heat termed “sensible heat”.
In case of super heated steam, steam has to first cool to saturation
temperature before it can condense to release its latent heat. The amount of
heat given up by superheated steam while reaching saturation temperature is
relatively small in comparison to the latent heat of evaporation.
Thus, as long as steam is super heated it has a lower heat transfer co-efficient
as maximum heat transfer occurs when saturated steam transfers its latent
heat.
Illustration :
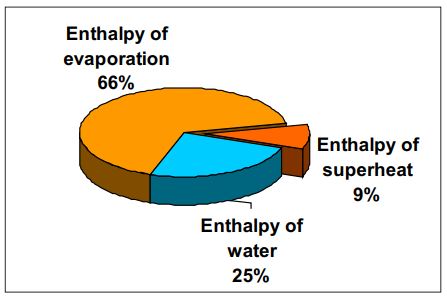
Heat energy contained in superheated steam is in the form of enthalpy of
water, latent heat / evaporation and superheat. The bulk of this energy is
enthalpy of latent heat / evaporation while the enthalpy of superheat forms the
smallest in proportion.
Consider superheated steam at 10 bar
g and 300 °C, then:
Enthalpy of water = 181 kcal/kg
Enthalpy of evaporation = 481.9 kcal/kg
Enthalpy of superheat = 65.7 kcal/kg
Superheated steam requires a larger
heat exchange area which, if not
provided, leads to reduced heat
exchange.