Importance of Steam Purity During Startups
Purity of steam at startup is one of important considerations for healthy and efficient operation of a steam
turbine. Chemical analysis of steam during startup and inspection of the turbine give a good indication of
steam quality.
Chemical analysis of steam should include measurement of sodium, anions and cations.
This ensures that no corrosive anions or other corrosive contaminants which can cause corrosion of turbine
blades are present in steam.
Main steam conductivity after cation exchange (CACE) values are monitored continuously during plant startups.
As specified in various international standards (IAPWS/ VGB/EPRI) steam should not be injected into the steam turbine unless the CACE value drops below 0.2 μS/cm.
Steam turbine startups are often delayed just to ensure that CACE values are within prescribed limits.
Increased CACE values during startup not only compromise safety of turbine operation, but also cause a
masking effect of dissolved CO2 which further delays plant startup.
Source of Carbon Dioxide in Condensate and Superheated Steam
Carbon dioxide is an impurity that enters the steam / water cycle mostly as air in-leakage or by decomposition
of organic matter (impurities or added treatment chemicals). Dissolved CO2 contributing to CACE values is
considered non-corrosive to the steam turbine when concentration is within reasonable limits
(e.g., CACE in steam <0.5 μS/cm).
However, an increase in CACE levels can mask the presence of other impurities like chlorides and anions, which
in turn can cause corrosion and failure. Differential analysis between cation and degassed conductivity
measurements can provide a more accurate cation conductivity value that can be directly correlated to CO2 concentration
and result in a more realistic assessment of process chemistry.
Principle for Cation Conductivity Measurement (CACE)
Cation conductivity measurement, as such, is a blind method of cross checking whether there are dissolved impurities present in the sample or whether it is ultrapure water. Ultrapure water is very poorly ionised and hence possesses extremely low conductivity. With the addition of even a small amount of salt (say NaCl), conductivity shoots up drastically. This can happen even with addition of desired chemicals as mentioned above. The typical reaction on the cation column will be :
1. Na(+) + Cl(-) => H(+) ions => HCl + Na(+) ions
2. NH4(+) + OH (-) =>. H(+) ions => H2O + NH4(+) ions
In the cation columns, resins present are charged with H(+) ions. These ions replace the +ve ions of any salt / dissolved impurity as it dissociates in water.
In case (1), the cations replace Na ions and the outcome is HCl, the corresponding acid.
In case (2), the cations replace NH4 ions and the result is water (H2O), i.e. pure water. The conductivity in case (1) is 3 times that imparted by the salt (here NaCl ), while in case (2), the conductivity imparted by the chemical (here NH4OH) gets eliminated as the outcome is pure H2O.
Thus cation conductivity measurement eliminates the masking effects of known/desired dosing chemicals (mainly ammonia).
Measuring Principle for Degassed Cation Conductivity (DCACE)
As explained above, the cation column removes effects of ammonia and other alkalizing agents, but still dissolves gases like CO2 which contributes to the CACE value. Carbon dioxide can form carbonic acid and carbonate on dissolution in water. This can severely affect not only cycle corrosion, but mask early condensate pH and conductivity measurements in cycle regions where condensation sets in. The most important paths for CO2 ingress are air and cooling water in leakage, makeup water, ion exchange resins, and plant cycle additives. By precise heating of the process solution to near-boiling after a cation column, carbon dioxide and other low molecular organics are volatised and removed.
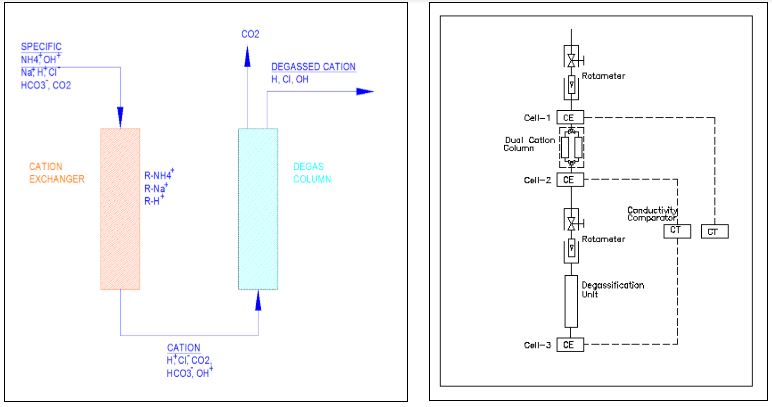
The figure above shows a typical layout for cation conductivity measurement. The conductivity is measured before the sample enters the cation column. This is called ‘specific conductivity’ measurement. The sample then flows through the cation column. The 3-way ball valve at the entry is used to pass the sample through the desired cation column (The duty column - say column-1 in this case). The position of the 3-way ball valve at the bottom also is selected in the same fashion. Column-2 can be selected when the resin in the first column gets exhausted.
Related Products
Degas Column Relisafe ™ CO²
