In a mixture of condensate and flash, although flash steam is only about 10% by mass it holds 50% of the energy content of condensate. Recover flash steam to utilize this energy and thus reduce fuel bills.
Condensate is discharged through steam traps from a high to a lower pressure. As a result some of this condensate evaporates into flash steam.
The quantity of flash steam generated depends on the amount of heat that can be held in condensate. Typically, 10% to 15% of the condensate is converted to flash steam.
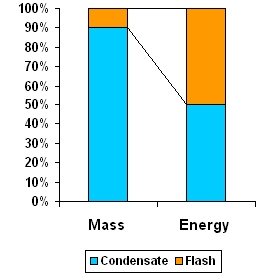
Illustration:
1 Kg of condensate at 4 bar g with a saturation temp of 1520C contains 152 kcal of heat. The condensate is discharged via a steam trap to atmosphere. But water at atmospheric pressure can only attain 990C with 99 kcal of heat. This means there is now an excess heat of 530C or 53kcal.
As per laws of thermodynamics, energy can neither be created nor be destroyed. This excess heat is used in boiling a portion of condensate into flash steam, thereby maintaining the energy balance.
1 kg of steam at atmospheric pressure has a total energy content of 638kcal. So 53kcal will boil 120gms of the 1kg of condensate into flash steam.
As seen, the energy converted to flash steam (53kcal) is more than half the energy in 1 kg of condensate (99kcal) but is only 12% of mass of condensate. Thus it is worthwhile to recover both condensate and flash steam.